Enhanced Aggregation of Alginate-Coated Iron Oxide (Hematite) Nanoparticles in the Presence of Calcium, Strontium, and Barium Cations
Kai Loon Chen, Steven E. Mylon, and Menachem Elimelech
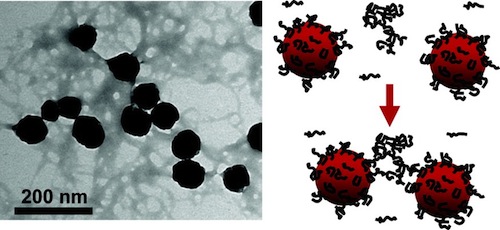
Early-stage aggregation kinetics studies of alginate-coated hematite nanoparticles in solutions containing alkaline-earth metal cations revealed enhanced aggregation rates in the presence of Ca2+, Sr2+, and Ba2+, but not with Mg2+. Transmission electron microscopy (TEM) imaging of the aggregates provided evidence that alginate gel formation was essential for enhanced aggregation to occur. Dynamic light scattering (DLS) aggregation results clearly indicated that a much lower concentration of Ba2+ compared to Ca2+ and Sr2+ was required to achieve a similar degree of enhanced aggregation in each system. To elucidate the relationship between the alginate’s affinities for divalent cations and the enhanced aggregation of the alginate-coated hematite nanoparticles, atomic force microscopy (AFM) was employed to probe the interaction forces between alginate-coated hematite surfaces under the solution chemistries used for the aggregation study. Maximum adhesion forces, maximum pull-off distances, and the work of adhesion were used as indicators to gauge the alginate’s affinity for the divalent cations and the resulting attractive interactions between alginate-coated hematite nanoparticles. The results showed that alginate had higher affinity for Ba2+ than either Sr2+ or Ca2+. This same trend was consistent with the cation concentrations required for comparable enhanced aggregation kinetics, suggesting that the rate of alginate gel formation controls the enhanced aggregation kinetics. An aggregation mechanism incorporating the gelation of alginate is proposed to explain the accelerated aggregate growth in the presence of Ca2+, Sr2+, and Ba2+.
The graduate student who initially built the ALV goniometer system in the lab of Meny Elimelech, Kai Loon Chen is now an Assistant Professor in the Johns Hopkins Whiting School of Engineering, Department of Geography and Environmental Engineering.
View additional papers from Kai Loon’s graduate career:
Relating Colloidal Stability of Fullerene (C60) Nanoparticles to Nanoparticle Charge and Electrokinetic Properties, by Chen and Elimelech. Environmental Science & Technology 43, 7270–7276 (2009). DOI: 10.1021/es900185p
Reduced Aggregation and Sedimentation of Zero-Valent Iron Nanoparticles in the Presence of Guar Gum, by Tiraferri, Chen, Sethi, and Elimelech. Journal of Colloid and Interface Science, 32471-79 (2008). DOI: 10.1016/j.jcis.2008.04.064
Enhanced Aggregation of Alginate-Coated Iron Oxide (Hematite) Nanoparticles in the Presence of Calcium, Strontium, and Barium Cations, by Chen, Mylon, and Elimelech. Langmuir,23 5920-5928 (2007). DOI: 10.1021/la063744k
Influence of Humic Acid on the Aggregation Kinetics of Fullerene (C60) Nanoparticles in Monovalent and Divalent Electrolyte Solutions, by Chen and Elimelech. Journal of Colloid and Interface Science, 309 126-134 (2007). DOI: 10.1016/j.jcis.2007.01.074
Aggregation and Deposition Kinetics of Fullerene (C60) Nanoparticles, by Chen and Elimelech. Langmuir, 22 10994-11001 (2006). DOI: 10.1021/la062072v
Aggregation Kinetics of Alginate-Coated Hematite Nanoparticles in Monovalent and Divalent Electrolytes, by Chen, Mylon, and Elimelech. Environmental Science and Technology,40 1516-1523 (2006). DOI: 10.1021/es0518068
Influence of Natural Organic Matter and Ionic Composition on the Kinetics and Structure of Hematite Colloid Aggregation: Implications to Iron Depletion in Estuaries, by Mylon, Chen, and Elimelech. Langmuir, 20 9000-9006 (2004). DOI: 10.1021/la049153g