March 10, 2017
Recent work in the Anastas lab has extracted metals from metal-rich waste using plants, in a process called phytoextraction, and produced a novel manganese-based catalyst called EcoMnOx. Chun Ho Lam and colleagues use DLS to characterize the resulting heterogeneous catalysts (nanoparticles) on the nanometer scale. This work has been published in ACS Sustainable Chemistry & Engineering.
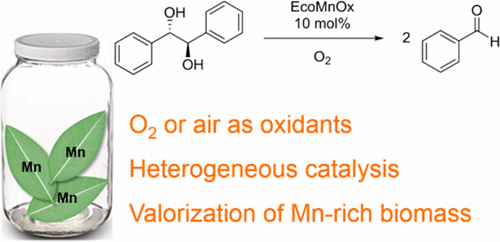
EcoMnOx, a Biosourced Catalyst for Selective Aerobic Oxidative Cleavage of Activated 1,2-Diols
Vincent Esconde, Chun Ho Lam, Claude Grison, Paul T Anastas
DOI: 10.1021/acssuschemeng.6b02979
Abstract:
A novel catalyst, EcoMnOx, was prepared from waste biomass of Mn-hyperaccumulating plants. The valorization of this Mn-rich biomass is an alternative to its costly usual disposing and provides a new source of Mn. Extracted metal ions, including Mn2+, were oxidized in mild conditions by H2O2/NaOH to afford EcoMnOx, as a polymetallic oxide material containing from 8.9 to 14.1 wt % Mn. Spectroscopic studies of this material revealed the presence of Mn layered mixed oxides, rich in MnIV along with MnIII and MnII species. EcoMnOx catalytic properties were assessed in the aerobic oxidative cleavage of 1,2-diols, under atmospheric pressure of O2 or air. With only 10 mol % Mn, up to complete conversions were obtained on activated benzylic and allylic diols, with excellent selectivity toward aldehydes or ketones (98–99%). Moreover, because of its heterogeneous nature, the catalyst can be removed easily by filtration, and reapplied for a minimum of six successive runs without any loss of activity. Finally, E-factor analysis showed the EcoMnOx generates 4 to 17 times less waste compared to classical reagents such as NaIO4 and Pb(OAc)4, respectively, highlighting the sustainable assets of this new heterogeneous catalyst.