Chemistry graduate student Julie Thomsen, in the lab of Gary Brudvig, and in collaboration with Yale’s Robert Crabtree and Ulrich Hintermair of Bath University, published her latest article in JACS, demonstrating the use of electrochemically activated water-oxidation catalysts from an organometallic iridium complex:
Electrochemical Activation of Cp* Iridium Complexes for Electrode-Driven Water-Oxidation Catalysis
Abstract:
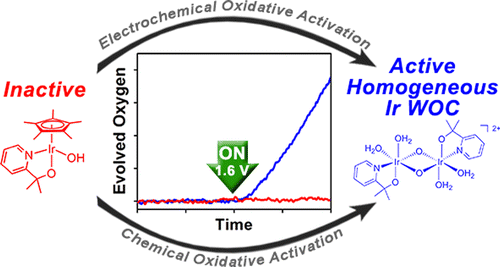
Organometallic iridium complexes bearing oxidatively stable chelate ligands are precursors for efficient homogeneous water-oxidation catalysts (WOCs), but their activity in oxygen evolution has so far been studied almost exclusively with sacrificial chemical oxidants. In this report, we study the electrochemical activation of Cp*Ir complexes and demonstrate true electrode-driven water oxidation catalyzed by a homogeneous iridium species in solution. Whereas the Cp* precursors exhibit no measurable O2-evolution activity, the molecular species formed after their oxidative activation are highly active homogeneous WOCs, capable of electrode-driven O2 evolution with high Faradaic efficiency. We have ruled out the formation of heterogeneous iridium oxides either as colloids in solution or as deposits on the surface of the electrode, and found indication that the conversion of the precursor to the active molecular species occurs by a similar process whether carried out by chemical or electrochemical methods. This work makes these WOCs more practical for application in photoelectrochemical dyads for light-driven water splitting.