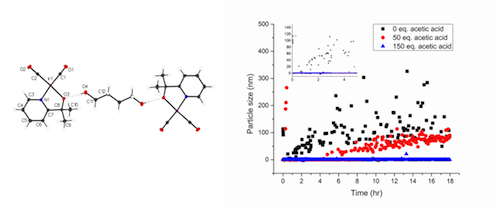
Daria Huang, a graduate student in the lab of Robert Crabtree in the Yale Department of Chemistry, in collaboration with Gary Brudvig and coworkers, recently introduced a new iridium precursor compound for the catalysis of water-oxidation reactions. The compound, IrI(CO)2(pyalc) (pyalc = (2-pyridyl)-2-propanoate), shown in the image, forms an oxidation catalyst upon introduction of a chemical oxidant to solution. Electrochemistry can also activate the compound. Of major interest in this work was the ability of the precursor complex to form a homogeneous catalyst, where the active species works in solution, rather than degrade into a heterogeneous catalyst like iridium oxide nanoparticles, which are generally less active than the solution-species. DLS studies confirm that the addition of acetic acid prevents the formation of iridium oxide nanoparticles from this compound. When activated in the presence of acetic acid, IrI(CO)2(pyalc) becomes a homogeneous catalyst of Ir(pyalc) isomers.
A new Ir bis-carbonyl precursor for water oxidation catalysis
Daria L. Huang, Rodrigo Beltrán-Suito, Julianne M. Thomsen, Sara M. Hashmi, Kelly L. Materna, Stafford W. Sheehan, Brandon Q. Mercado, Gary W. Brudvig, Robert H. Crabtree
DOI: 10.1021/acs.inorgchem.5b02809
Abstract:
This paper introduces the IrI(CO)2(pyalc) (pyalc = (2-pyridyl)-2-propanoate) as an atom-efficient precursor for Ir-based homogeneous oxidation catalysis. This compound was chosen to simplify analysis of the water oxidation catalyst species formed by the previously reported Cp*IrIII(pyalc)OH water oxidation precatalyst. Here, we present a comparative study on the chemical and catalytic properties of these two precursors. Previous studies show that oxidative activation of Cp*Ir-based precursors with NaIO4 results in formation of a blue-colored IrIV species. This activation is concomitant with the loss of the placeholder Cp* ligand which oxidatively degrades to form acetic acid, iodate, and other obligatory by-products. The activation process requires substantial amounts of primary oxidant, and the degradation products complicate analysis of the resulting IrIV species. The species formed from oxidation of the Ir(CO)2(pyalc) precursor , on the other hand, lacks these degradation products—the CO ligands are easily lost upon oxidation—which allows for more detailed examination of the resulting Ir(pyalc) active species both catalytically and spectroscopically, although complete structural analysis is still elusive. Once Ir(CO)2(pyalc) is activated, the system requires acetic acid or acetate to prevent the formation of nanoparticles. Investigation of the activated bis-carbonyl complex also suggests several Ir(pyalc) isomers may exist in solution. By 1H NMR, activated Ir(CO)2(pyalc) has fewer isomers than activated Cp*Ir complexes, allowing for advanced characterization. Further research in this direction contributes toward our structural understanding of the active species.