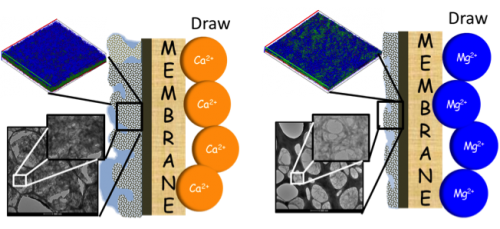
Advancements in membrane filtration technology promise to improve access to clean water throughout the world. However, technical issues such as biofouling of membranes due to bacterial contamination remain a key challenge. In this work, post-doc Ming Xie and colleagues from the Elimelech lab investigate the effect of magnesium and calcium salts on the biofouling of forward osmosis membranes. Published in ES&T, the study shows that membranes are more susceptible to biofouling from extracellular polymeric substances in the presence of calcium than magnesium.
Role of Reverse Divalent Cation Diffusion in Forward Osmosis Biofouling
Ming Xie, Edo Bar-Zeev, Sara M. Hashmi, Long D. Nghiem, and Menachem Elimelech
Abstract:
We investigated the role of reverse divalent cation diffusion in forward osmosis (FO) biofouling. FO biofouling by Pseudomonas aeruginosa was simulated using pristine and chlorine-treated thin-film composite polyamide membranes with either MgCl2 or CaCl2 draw solution. We related FO biofouling behavior – water flux decline, biofilm architecture, and biofilm composition – to reverse cation diffusion. Experimental results demonstrated that reverse calcium diffusion led to significantly more severe water flux decline in comparison with reverse magnesium permeation. Unlike magnesium, reverse calcium permeation dramatically altered the biofilm architecture and composition, where extracellular polymeric substances (EPS) formed a thicker, denser, and more stable biofilm. We propose that FO biofouling was enhanced by complexation of calcium ions to bacterial EPS. This hypothesis was confirmed by dynamic and static light scattering measurements using extracted bacterial EPS with the addition of either MgCl2 or CaCl2 solution. We observed a dramatic increase in the hydrodynamic radius of bacterial EPS with the addition of CaCl2, but no change was observed after addition of MgCl2. Static light scattering revealed that the radius of gyration of bacterial EPS with addition of CaCl2 was 20 times larger than that with the addition of MgCl2. These observations were further confirmed by transmission electron microscopy imaging, where bacterial EPS in the presence of calcium ions was globular, while that with magnesium ions was rod-shaped.