Researchers in James Mayer’s lab in the Yale Chemistry Department have recently used dynamic light scattering and electrophoretic mobility measurements to elucidate the nature of charge transfer in titanium dioxide nanoparticles. By carefully preparing and characterizing particles of only a few nanometers in size, lead author Jennifer Peper and coworkers reveal important considerations in the design of nanomaterials for applications including solar energy and photocatalysis. This work has been published in JACS.
Slow Equilibration between Spectroscopically Distinct Trap States in Reduced TiO2 Nanoparticles
Jennifer L Peper, David J. Vinyard, Gary Brudvig, James Mayer
Abstract:
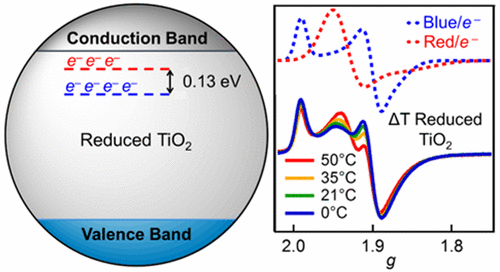
Understanding the nature of charge carriers in nanoscale titanium dioxide is important for its use in solar energy conversion, photocatalysis, and other applications. UV-irradiation of aqueous, colloidal TiO2 nanoparticles in the presence of methanol gives highly reduced suspensions. Two distinct types of electron traps were observed and characterized by EPR and optical spectroscopies. The relative populations of the states depend on temperature, indicating a small energy difference, ΔH° = 3.0 ± 0.6 kcal/mol (130 ± 30 meV). Interconversion between the electron traps occurs slowly over the course of minutes to hours within the temperature range studied here, 0–50 °C. The slow time scale implies that interconversion involves changes in structure or stoichiometry, not just the movement of electrons. This occurrence of slow structural modification with changes in trap state occupancy is likely a general feature of reduced TiO2 systems at thermodynamic equilibria or photostationary states and should be considered in the design of TiO2-containing devices.